
Applied Clinical Trials
Over the past year and a half we have been monitoring the trend towards adoption of risk-based monitoring across the industry, and in particular the move towards reduced source document verification (SDV).

Applied Clinical Trials
Over the past year and a half we have been monitoring the trend towards adoption of risk-based monitoring across the industry, and in particular the move towards reduced source document verification (SDV).

Applied Clinical Trials
GLOBAL TRIALS : Is Guatemala the Next Research Hotspot? SITES : Trials in Limited Resource Settings Also in this issue : New User Fees, Declining European Trials, Low Trial Results Reporting, Risk-Based Approach to Monitoring

Applied Clinical Trials
Pressure to approve new user fees will affect policies for foreign studies and research methods.

Applied Clinical Trials
The responsibilities regarding CV safety for drug developers are constantly evolving.
Applied Clinical Trials
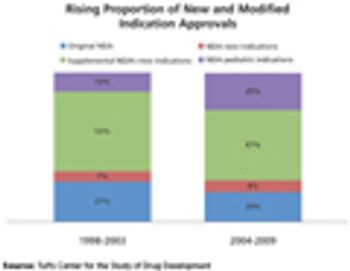
Tufts Center for Drug Development

Applied Clinical Trials
While risk and uncertainty remain high, the pace of innovation will remain glacial.
Applied Clinical Trials
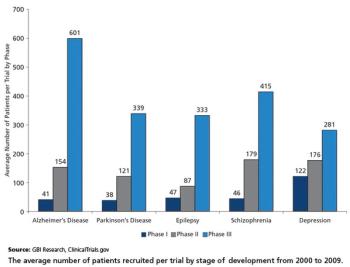
Often CNS trial subjects face unique obstacles like mental dependency

Applied Clinical Trials
Examining the challenges and solutions to the implementation of trials in resource-limited settings.

Applied Clinical Trials
The importance of safe use of imaging agents was highlighted at the Chicago Radiological Society of North America conference.

Applied Clinical Trials
A case study of how cardiac safety assessments can be enhanced with Holter Bin.

Applied Clinical Trials
Why biotechs need to use a more systematic approach and catch up with pharma.

Applied Clinical Trials
Compliance with reporting clinical trial results is low, and an even larger opportunity is being missed.

Applied Clinical Trials
Clinical R&D organizations across the industry have been steadily moving away from the traditional approach to site monitoring using 100% source document verification (SDV) of subject eCRFs, and into a new paradigm where a targeted sampling of the subject eCRF data is verified against source charts.

Applied Clinical Trials
Patricia Brunko addresses the decline in European clinical trials, but still gives the industry hope.
Applied Clinical Trials
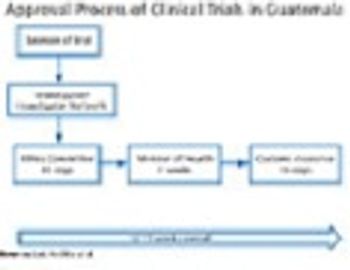
Guatemala is multi-ethnic, multilingual, and multi-cultural-ideal for international trials.

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.

Applied Clinical Trials
Benefits of centralized cardiac safety services as seen in a study for treatment of allergic rhinitis.