
Gilead’s Biktarvy is now the first and only INSTI-based single-tablet regimen that is FDA approved and DHHS guideline recommended for people who are virologically suppressed with M184V/I resistance.

Gilead’s Biktarvy is now the first and only INSTI-based single-tablet regimen that is FDA approved and DHHS guideline recommended for people who are virologically suppressed with M184V/I resistance.
Survodutide produced a statistically significant improvement in metabolic dysfunction-associated steatohepatitis among 83% of those administered the drug compared to 18.2% of those given a placebo.

Biopharma company XYRA and FDA have concluded a series of Phase II meetings on managing key studies for potential atrial fibrillation therapy, budiodarone.

In an interview with ACT Editor Andy Studna, Gadi Saarony, CEO of Advarra talks about using technology to better diversify clinical trials and what to expect in the new year.

Phase Ia, open-label, escalating dose study to evaluate the safety and immunogenicity of therapeutic vaccine, TherVacB.
Trial data show Dupixent rapidly and significantly improved lung function compared to placebo in adults with uncontrolled chronic obstructive pulmonary disease with type 2 inflammation.

Agency reports concerns amid an increase in submitted data that has been fabricated, duplicated, or unreliable from third-party laboratories.

In an interview with ACT Editor Andy Studna, Gadi Saarony, CEO, Advarra, offers his thoughts on the status of the pharmaceutical industry improving diversity in clinical trials.

Enrollment and initial follow up has been completed for study set to test efficacy of cancer vaccine in individuals with Lynch syndrome.

An overview of patient perceptions of cancer clinical trials and the consent process among those who are currently enrolled in, or who recently completed, a cancer clinical trial.

Though artificial intelligence has yet to achieve its full potential, meaningful strides are still being made across the drug discovery funnel.

Clinical-stage biotech’s lead drug candidate, PrimeC for the treatment of ALS demonstrated a clinically meaningful effect on quality of life and on complication free-survival for patients.
Ocifisertib has shown significant single-agent activity in both solid and liquid tumors.

ARV-102 is a novel oral PROTAC protein degrader designed to cross the blood-brain barrier and target leucine-rich repeat kinase 2.

Upgrades include end-of-life equipment replacements and relocation.
The Phase I/II MajesTEC-1 trial (NCT03145181; NCT04557098) evaluated Tecvayli (teclistamab-cqyv) at a reduced dose of 1.5 mg/kg administered every two weeks in patients with relapsed/refractory multiple myeloma.

Phase II GenePHIT trial is evaluating the safety and efficacy of a single intracoronary infusion of AB-1002.
Data from the pivotal, global, randomized, double-blind, placebo-controlled, Phase III INDIGO trial show statistically significant and clinically meaningful progression-free survival for vorasidenib treating IDH-mutant gliomas.
Trial findings show Krazati (adagrasib) plus cetuximab was well tolerated with promising clinical activity among pretreated patients with locally advanced or metastatic colorectal cancer harboring a KRASG12C mutation
Datopotamab deruxtecan, the first TROP2-directed DXd antibody drug conjugate, is being evaluated for locally advanced or metastatic nonsquamous non-small cell lung cancer.

In an interview with ACT editor Andy Studna at SCOPE, Corrigan discusses what YPrime is doing to address challenges in clinical trials and results from the company's recently released eCOA report.

It is crucial throughout the clinical trials space to understand the challenges and triumphs of DEI.
Partnership comes amid two promising Phase III trial results for non-opioid, non-addictive medication for fibromyalgia.
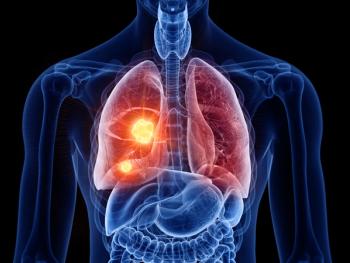
Results from the randomized, multicenter, open-label, Phase III FLAURA 2 trial show Tagrisso plus chemotherapy delayed disease progression by nearly nine additional months in adults with locally advanced or metastatic epidermal growth factor receptor-mutated non-small cell lung cancer.
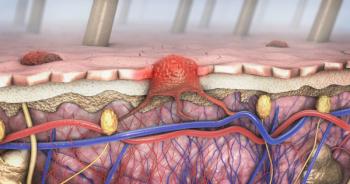
Amtagvi (lifileucel) is a personalized, one-time therapeutic option for adult patients with unresectable or metastatic melanoma who received prior treatment with a PD-1 antibody, and in patients who are BRAF V600-positive, a BRAF inhibitor with or without a MEK inhibitor.

In clinical trials, Xolair was found to significantly increase the amount of peanuts, milk, egg, and cashew that it takes to trigger an allergic reaction in participants with food allergies.


Single-arm, open-label, multicenter, non-randomized, multicohort Phase II VISION trial (NCT02864992) of Tepmetko (tepotinib) for metastatic non–small cell lung cancer shows favorable objective response rate.

A recent study showed that a multimodal machine learning approach accelerated the time in which investigators could determine the efficacy of sertraline treatment in major depressive disorder

Many companies have implemented new technologies and are adopting clinical trial models that are more patient-centric.