With $13 million grant, Vanderbilt University Medical Center will lead the new multisite trial.

With $13 million grant, Vanderbilt University Medical Center will lead the new multisite trial.
Casgevy is a non-viral, ex vivo CRISPR/Cas9 gene-edited cell therapy that was also recently approved for sickle cell disease.
A pair of clinical trials show the rapid and reliable efficacy of Zoryve in treating atopic dermatitis and seborrheic dermatitis.

New analysis for 2023 shows signs of post-pandemic recovery; breast cancer remains most studied disease area.

Because artificial intelligence use in healthcare is powered by patient data, it is imperative to guard privacy by verifying that security measures are in place to protect against any data breaches.
Data from the Phase III LIBERTY-AD-HAFT trial show 40% of patients with atopic dermatitis with uncontrolled moderate-to-severe hand and/or foot involvement who were administered Dupixent achieved clear or almost clear skin on their hands and feet.

Scaling seven common digital programs inside a typical top 10 pharma company could be worth $1.4 billion to $2.2 billion in operating income over five years.

The 39th overall approval for Keytruda was based on data from the Phase III KEYNOTE-A18 trial in patients with FIGO 2014 stage III to IVA cervical cancer.
Study examines how many registered clinical trials with published protocols are also publishing their results.
An open-label, randomized, dose-optimization Phase II trial (NCT06173037) is assessing the antibody-drug conjugate RC88 to determine the optimal dosage, efficacy, and safety in patients with platinum-resistant ovarian cancer.
The efficacy of Axon Therapy was shown in a randomized controlled trial that demonstrated significant efficacy in reducing pain and numbness associated with painful diabetic neuropathy.

The revision of Annex 1 clearly calls out the use of quality risk management to identify potential risks to quality and the implementation of a contamination control strategy.

Clinical study identifies combination of 11 proteins that can predict long-term disability in patients with multiple sclerosis.
SLS009 is a CDK9 inhibitor under evaluation in an ongoing Phase I/II study in patients with relapsed or refractory acute myeloid leukemia.

Shorla Oncology’s New Drug Application for SH-105 for breast and ovarian cancers was given a Prescription Drug User Fee Act action date of June 29, 2024.
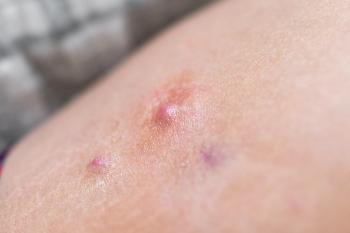
AbbVie will advance the clinical program for the investigational, dual-variable-domain IL 1α/1β antagonist lutikizumab to Phase III for the treatment of adults with moderate to severe hidradenitis suppurativa who previously failed anti-TNF therapy.

Utilizing known processes and managing risks are among best practices with new technologies, such as artificial intelligence, according to Gottlieb.

The FDA's complete response letter was not related to clinical trial data for efficacy or safety for zolbetuximab for the treatment of patients with locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction.

Findings from the Phase III innovaTV 301 trial showed a favorable benefit/risk profile for Tivdak (tisotumab vedotin-tftv), as well as improved overall survival for patients with recurrent and metastatic cervical cancer who have limited treatment options.
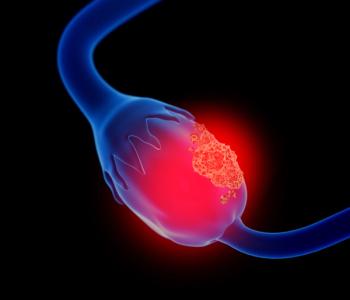
Rinatabart sesutecan (Rina-S; PRO1184) is a novel folate receptor alpha (FRα)–targeted antibody-drug conjugate for patients with FRα-expressing high-grade serous or endometrioid platinum-resistant ovarian cancer.

Expanding clinical trial access to include historically underrepresented ethnic and racial minorities means addressing root-cause barriers, including taxation.

Study highlights efficacy of effectively monitoring attention-deficit hyperactivity disorder treatment effects with Qbtest.
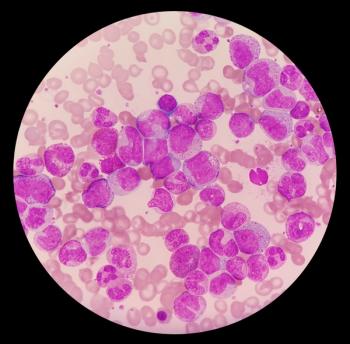
Phase III ASC4FIRST show Scemblix (asciminib) plus investigators’ choice of tyrosine kinase inhibitor produced a statistically significant response in patients newly diagnosed with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase.
The use of modeling and biosimulation can help predict potential outcomes and improve confidence in therapeutic candidates for Alzheimer disease.
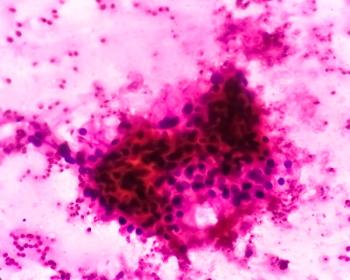
Data from the Phase III ZIRCON trial show the positron emission tomography imaging agent 89Zr-DFO-girentuximab was more effective than traditional PET/CT imaging in identifying malignant renal cell carcinoma lesions.

Merck is actively enrolling patients for investigational drugs that treat essential thrombocythemia, chronic lymphocytic leukemia, small lymphocytic lymphoma, non-small cell lung cancer, endometrial carcinoma, and metastatic castration-resistant prostate cancer.

Results from the Phase III BOND-003 trial demonstrated that treatment with CG Oncology Inc’s cretostimogene grenadenorepvec (CG0070) provided clinical benefit in complete responses with acceptable tolerability for the treatment of patients with high-risk Bacillus Calmette-Guérin (BCG)–unresponsive non–muscle invasive bladder cancer.

DCTs, data, and analytics are all areas that could be impacted in the near future.

Effects of newly implemented NIH policy on data sharing could be profound for clinical research.
EUA submission based on positive initial findings from the pivotal Phase III CANOPY clinical trial for VYD222, a broadly neutralizing, half-life extended monoclonal antibody developed specifically to prevent COVID-19 in immunocompromised adults and adolescents.