
The Partnerships in Clinical Trials Conference was a large venue that attracted many high-level executives from biopharmaceutical companies

The Partnerships in Clinical Trials Conference was a large venue that attracted many high-level executives from biopharmaceutical companies
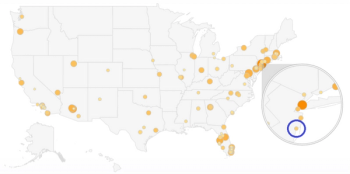
This article will describe my experiences in creating robust strategies for optimizing site selection and offer a few recommendations.

An interview with Dr. Greg Koski about the Alliance for Clinical Research Excellence and Safety (ACRES).
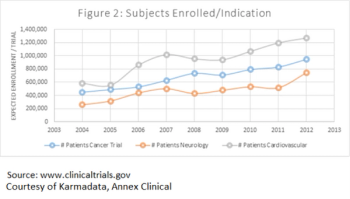
Scientists and experts have conducted extensive research on protocol optimization and the need to enhance study efficiency, and sponsors are starting to look at their study design strategies.
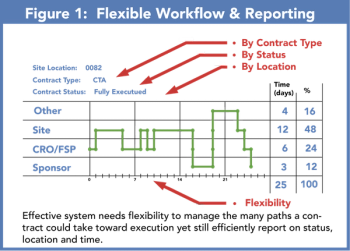
The management and oversight of clinical trials agreement contracts are still handled through spreadsheets or document management systems ill equipped for an efficient CTA lifecycle.

Patients communicated alarming behaviors clearly attributed to a lack of communications and engagement from study teams.

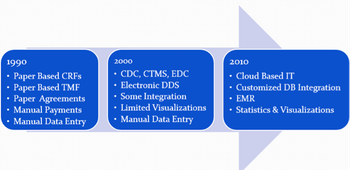
The clinical trial technological landscape continues to morph, as we are starting to see the emergence of novel technologies and techniques to enhance clinical trial strategies and productivity.

With increasing challenges surrounding clinical trial subject enrollment and engagement, the landscape of clinical trials continues to morph.

Many biopharmaceutical enterprises and CROs are trying to establish solid risk assessment techniques and infrastructures to enhance clinical trial data quality, strategy, and reduce monitoring costs
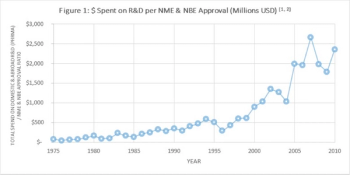
Many of us concur that the biopharmaceutical industry is facing a crisis, and that the R&D process needs to transform entirely in order to address skyrocketing costs and minimal effectiveness.

Given FDA's new guidance on risk-based monitoring (RBM), there has been a lot of interest on identifying critical data points, drafting RBM plans, and executing RBM.

Over the past several years, we have been seeing an influx of significant changes in the clinical trials industry ranging from changes in the way we approach clinical trials, and massive growth in new technologies.

Due to increasing costs and lacking productivity of the existing clinical trial operational model, there has been a lot of awareness around ?Future Clinical Trials,? and some sponsors are seeming to transform pharmacies into study sites.

In August of 2013, the FDA released a report that analyzed clinical trial subject demographic subgroups for FDA approved medical products, which included factors such as race, age and gender.

With the release of FDA's Guidance on Risk-Based Monitoring (RBM), many clinical operations personnel have been asking how they could implement RBM strategies.

Accessing and engaging patients is a very difficult task in today’s environment, as there is a bombardment of numerous media vehicles, including digital media, with varying intensities that penetrate patients’ senses, emotions, and thoughts.

With the increasing challenges associated with bringing a medical product to market, and skyrocketing R&D costs, there has been a shift in biopharmaceutical commercialization and clinical trial strategies in order to minimize risk and maximize success.





Increasing global competition from generics and a glut of expiring patents have forced many biopharmaceutical and medical device companies to rethink their operational strategies.