Moe Alsumidaie
Articles by Moe Alsumidaie

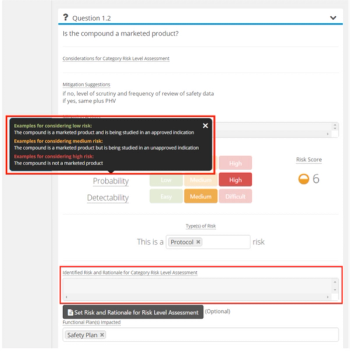
TransCelerate’s Risk Assessment and Categorization Tool (RACT) has exhibited usefulness with study teams and has guided organizations, such as Cancer Research UK (CRUK) to adopt their own RBM questionnaires

The ability to have access to real-time information through leveraging technology combined with a strategic business perspective allows companies to implement a true quality management system.

Applied Clinical Trials is collaborating with the Clinical Endpoints Adjudication Group to conduct an industry survey to evaluate the impact of technology on endpoint adjudication processes.
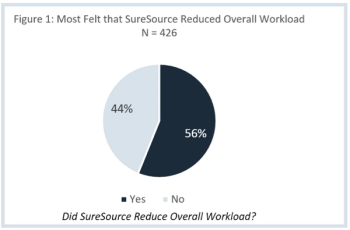
Clinical Ink, an eSource solutions provider, offered Applied Clinical Trials raw data from its Site Impact Survey for analysis. This article will delve into this data to uncover breakthrough trends regarding the utilization of eSource on workload reduction at study sites.
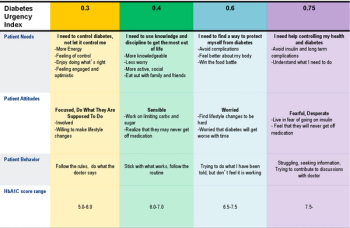
The topic of patient centricity remains very active in the clinical trials industry, as the concept continues to emerge and evolve. The purpose of this Patient Centered Clinical Trials facilitated by our friends at eyeforpharma virtual roundtable is to (a) better understand the definition of patient centricity, (b) uncover challenges associated with industry wide patient centered technology adoption and (c) how patient centricity/engagement technologies will push the biopharmaceutical industry to c

TransCelerate's Quality Management System (QMS) Initiative delineates specific mechanics that will be used to build a QMS Conceptual Framework to facilitate and encourage proactive approaches towards executing Good Clinical Practices, and proper change management expected to support a culture of quality.
Democratic candidate Hillary Clinton is introducing her Prescription Drug Plan which is meant to benefit patients, however, will Hillary’s plan be good for advancing novel therapies, and will it benefit R&D organizations?

IBM and ICON plc recently announced a collaboration to use the Watson system to enhance oncology clinical trial feasibility, recruitment and start up via Trial Matching.

The world of RBM is continually changing as biopharmaceutical enterprises are dabbling into different approaches and methods. There are several outsourced models that exist, when approaching RBM, such as integrating cloud-based solutions to provide centralized monitoring teams with analytics, or fully outsourcing RBM functions and technologies to CROs. However, some companies, such as Novartis, appear to be in-sourcing their RBM technologies and functions.

An article recently appeared in Applied Clinical Trials, which indicated that oncology trials are taking longer to complete, and the article suggested that the duration of oncology clinical trials in certain phases increased by one year to one and a half years.1 Moreover, the article delineates that ameliorations in trial duration were most likely a result of increasing protocol complexi

Clinical trial recruitment and enrollment has presented its challenges in many ways. For Quintiles’ Phase I Unit in Kansas, clinical trial recruitment was a matter of process and strategy that it improved with innovation and mobile health (mHealth) technology applications. This article and case study will delineate the challenges that Quintiles’ Phase I Unit underwent, and what it did to achieve transformative results in recruitment, study visit adherence, and operational efficiency.

This article will describe my experiences in acquiring new clinical trials from the study site’s standpoint.

Ben Rotz, Director of Medical Transparency at Eli Lilly & Company discusses TransCelerate’s Data Transparency Initiative and position paper regarding patient data de-Identification and anonymization.

The aforementioned article emphasized that a reduction in data quality ultimately leads to enrolling more clinical trial subjects in order to maintain equivalent statistical power.
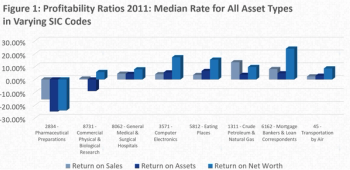
Optimizing study protocols and improving data quality have been notable areas of focus in the biopharmaceutical industry; the total number of endpoints in a typical Phase III protocol increased from 7 to 13.

The introduction of easily worn biosensors, such as FitBit, Garmin’s Vivofit, and Apple’s iWatch, have created a large influx of interest in the area of mobile health (mHealth).

Changes in biopharmaceutical R&D strategies and Technological innovations in clinical research are challenging payers on evaluating novel therapeutics and their involvement in the R&D process. While at the 2015 New York BIO annual convention, I had the opportunity to interview Nathan Tinker, Executive Director at the New York Biotechnology Association about how these changes are impacting payers.

TrialReach is a patient recruitment/enrollment technology and service that uses machine learning algorithms, in combination with patient networks to get eligible patients to the right trials.

Many of us speak about the importance of clinical trial data quality and integrity, yet the lack of data quality standards and definitions introduces subjectivity risk in clinical trials.

With the introduction of mobile health technologies in the field of healthcare, Sponsors and CROs are looking into mHealth to design patient centric clinical trials in order to reduce study visit costs and trial participation burden on patients.



With the clinical trial industry’s focus on improving monitoring efficiency and leveraging a variety of risk-based monitoring (RbM) models, novel eClinical technologies are emerging to facilitate clinical trial monitoring productivity.
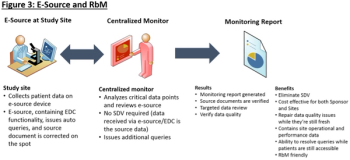
The emergence of risk-based monitoring (RbM) is creating a revolution in the way biopharmaceutical sponsors and CROs manage clinical trial quality.

The need for robust and standardized psychiatric outcomes measures in oncology trials has grown immensely
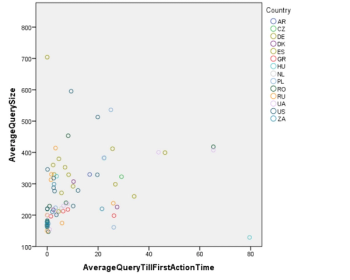
Clinical trial site engagement has been advocated as a critical component relating to a study’s performance and success, however, a minimum amount of data supports this connection.
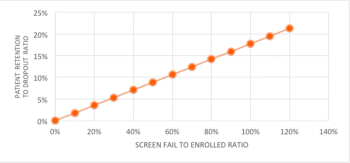
Clinical trial patient retention and dropout continues to be an issue amongst biopharmaceutical sponsors, as patient dropouts minimize the statistical power of clinical trial data, requiring study teams to enroll additional patients.
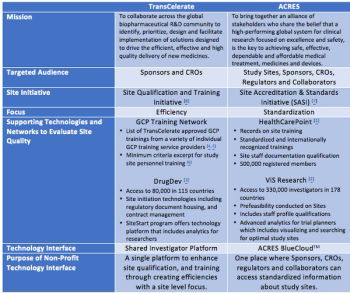
Over the past several years, there has been a rise in nonprofit organizations that focus on addressing the challenges in clinical research.

Patient adherence is an emerging topic and a matter of concern in clinical research. It applies to several different clinical trial facets.