What leads to Patient dropout? Read more here.

What leads to Patient dropout? Read more here.

The concept of risk-based monitoring (RbM) is evolving, as nonprofit organizations continue to collaborate with the biopharmaceutical/medical device industry to investigate, pilot and implement RbM practices.

The need for innovation in clinical trials has sparked significant interest from a variety of new enterprises including technology, analytics, subject enrollment/engagement/retention, EDC, risk-based monitoring, and many mor
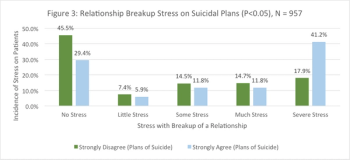
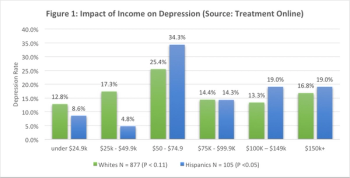
This article will analyze factors that affect suicidal ideation from a clinical psychiatric pilot study.

This guidance document will offer information about RbM strategies.
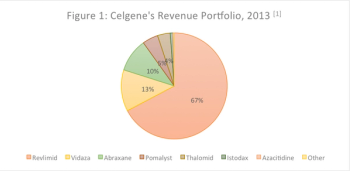
This article will analyze the impact of Revlimid on a different outcome: multiple myeloma mortality rates in the US.
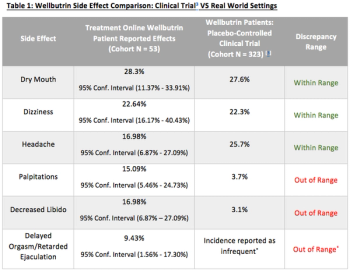
This article will evaluate some concerns regarding psychiatric clinical trial design, and will analyze post-marketing patient reported outcomes data on the antidepressant, Bupropion HCL (Wellbutrin), from Treatment Online, an online psychiatric treatment platform.

While the issue of adverse drug reaction (ADR) reporting is apparent in hospital systems, a deeper and uninvestigated problem resides within the process of ADRs: the patient.
There is a lot of discussion and awareness on the topic of patient centricity, as many experts are starting to discuss how the concept can improve clinical trials.

I recently visited the Caribbean isles, and on my way at the airport, I noticed prominent CDC disclaimers about an equatorial viral disease: Chikungunya.
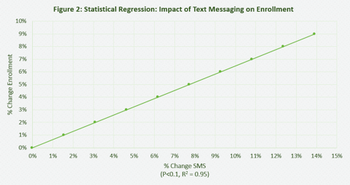
There is a lot of new research that evaluates the impact of Short Messaging System (SMS, Text Messages) in healthcare settings.
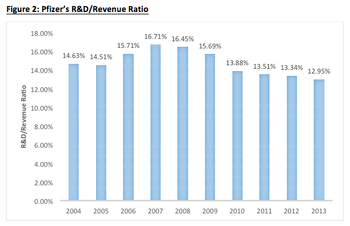
Is Pfizer prime for acquiring another biopharmaceutical company?
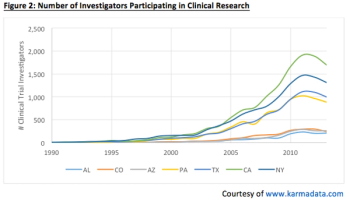
There are a number of indicators that suggest that US clinical trial growth is far from plateauing, and demand for clinical research investigators will continue to rise with this growing trend.
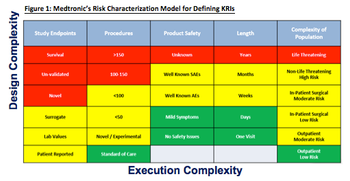
The industry has tried to establish some consistency by standardizing RBM methodologies.

Two main themes arose from the ePatient Connections Summit: online patient engagement and patient centricity.

Eye for Pharma hosted its first Patient Centered Clinical Trials symposium in Boston.

While at the 2014 NYBIO conference, Dr. Sam Waksal from Kadmon had the opportunity to address the audience regarding the state of the biopharmaceutical industry.
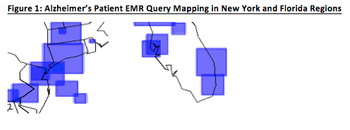
The topic of subject enrollment has been evaluated from many different perspectives.

This article will discuss FDA's post-marketing surveillance programs, and how biopharmaceutical sponsors can work with the FDA to identify unreported Adverse Events.

DIA held its 2014 annual meeting in San Diego and attracted thousands of attendees from around the globe.

While at the 2014 New York BIO conference, FDA Commissioner, Dr. Margaret Hamburg, had the opportunity to address pressing issues affecting the biopharmaceutical industry and the FDA.

While at the 2014 New York BIO Conference, I had the opportunity to interview Nathan Tinker, Executive Director at the New York Biotechnology Association, about challenges in early phase development.

Clinical trial optimization through adaptation in several key areas was a prevailing message at the recent Partnerships Conference.

Investments, acquisitions in biotechnology, valuation, and regulatory innovation were main themes at the 2014 NewYorkBIO Conference.
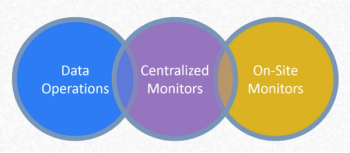
Thomas Verish, Group Director of Data Operations Services at Bristol-Myers Squibb, elaborated on their risk-based monitoring pilot.

While at the 2014 Bio IT World conference, I had the opportunity to interview Pek Lum, Ayasdi's Chief Data Scientist.

There is plenty of evidence and research which suggests that pharmacies are an excellent medium to engage clinical trial subjects.
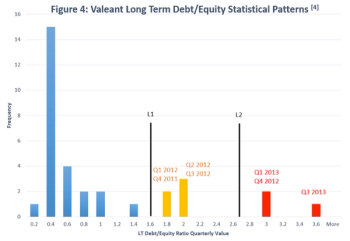
Douglas Tsao, Vice President at Barclays, spoke on the outcomes of Merges & Acquisitions, and its impact on company stock prices.
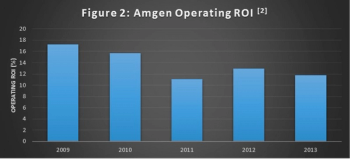
While at the 2014 Partnerships in Clinical Trials Conference, Adrian Otte, VP of Global Development Operations at Amgen, spoke about how Amgen's Risk-Based Monitoring and FSP models impacted business outcomes.

While the biopharmaceutical industry faces challenges in regulation, standardization, and change, CROs are often in the middle of the change.