
We explore the remote trial and hybrid models in clinical trial patient centricity with Hassan Kadhim, an executive at Boehringer Ingelheim.

We explore the remote trial and hybrid models in clinical trial patient centricity with Hassan Kadhim, an executive at Boehringer Ingelheim.

The Clinical Endpoints Adjudication Group and Ethical Clinical designed and implemented an industry survey to evaluate factors that drive the use of endpoint adjudication in clinical trials.

Takeda’s Atul Mahableshwarkar offers insight into central nervous system drug development and how biomarkers can improve therapy development of this discipline.

The challenges of data quality are a constant factor in clinical trials, especially that of traditional paper source documenting. Joyce Smith of The Medical Research Network speaks about the study’s site perspective on trial data quality.

Our series on patient centricity continues with a patient perspective on her interpretation of this subject. Shelly Hoover, an ALS patient, shares her views with Moe Alsumidaie.

Issues of counterfeit drugs being introduced into a supply chain have been a concern in comparative clinical trials. Terry Walsh of GSK speaks about the addressing this issue and ensuring that comparator drug supplies are more readily available for comparative trials.

The recent partnership between Continuum Clinical and Lyft has introduced the convenience of transportation into the hands of patients. Nariman Nasser, VP of Site Optimization at Continuum Clinical, elaborates on this partnership.
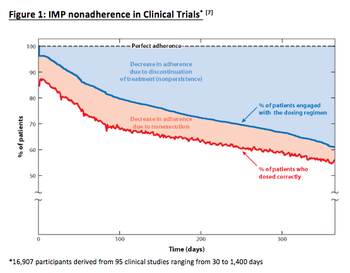
As clinical trials remain costly and continue to increase, the promise of novel initiatives gives hope that trial duration and cost impact will drop. However, biopharma continues to overlook one element that affects study timelines: patient non-adherence.

The adoption of electronic health records by hospitals and providers has increased engagement in Health Information Technology (HealthIT) activities. Nora Belcher of the Texas eHealth Alliance speaks on how HealthIT will change the pharma landscape for drug development.

The adoption of electronic health records by hospitals and providers has increased engagement in Health Information Technology (HealthIT) activities. Nora Belcher of the Texas eHealth Alliance speaks on how HealthIT will change the pharma landscape for drug development.

In continuing our series on patient centricity, we are adding the digital perspective from inside of a biopharmaceutical enterprise. In this interview with Jeremy Sohn, VP, Global Head of Digital Business Development & Licensing at Novartis, we will delve into how biopharmaceutical enterprises are incorporating digital strategy into patient centric study execution, and elaborate on some of the cultural challenges of adopting novel study methods.

Jeremy Sohn, VP, Global Head of Digital Business Development & Licensing at Novartis, delves into how biopharma is incorporating digital strategy into the execution of patient centric studies.

Meghan McKenzie, Associate Director and Sr. Clinical Program Lead at Genentech, elaborates on her experiences in the field of patient centricity from the perspective of the sponsor.

John Reites, formerly of Quintiles, and current Chief Product Officer at THREAD Research, discusses how mHealth is incorporated in clinical trial patient centricity.

We previously covered the challenges that biopharmaceutical enterprises are facing when it comes to developing CNS medical products at ExL’s 2nd CNS Clinical Trial Forum. In this interview, Glenn Morrison, Executive Director of Clinical Development at Zogenix, will discuss how biopharmaceutical enterprises can apply techniques used in oncology clinical trials in CNS development.

This survey on Endpoint Adjudication evaluates how this concept is impacting study design, uncovering operational challenges and determining outcomes.

Justin Stark, Head and Director of Risk-based Monitoring and Standards are UCB discusses advancements in RBM and TransCelerate’s Quantitative Metrics Toolkit.

As pharma continues to struggle with designing studies that result in creating breakthrough medical therapies, biopharma sponsors focusing on CNS studies face similar challenges and risks.
Nadir Benouali of MEDICODOSE Systems speaks on his experiences with investigational product nonadherence, its place in pharma and patient-centric ways to address it.
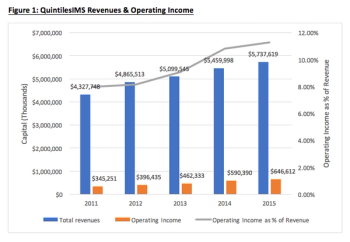
The CRO industry is experiencing exponential growth as a result of higher demand from the biopharma industry and an increased investment in R&D. This evaluation of QuintilesIMS attempts to provide some hints as to how this sector is advancing in the biopharma industry.

The standardization of trial metrics offers numerous benefits towards overseeing clinical trials, optimizing clinical operations and mitigating study risks. However, on a situational basis customized metrics may become necessary to address study-specific risks, uncover unknown issues and demonstrate results.

With much happening in the clinical trials industry during 2016, Moe Alsumidaie looks back at some of the innovations that took place. He also looks forward to what the industry can bring us in 2017.

The FDA’s Dr. John Whyte recently shared his perspectives on patient centricity at eyeforpharma’s Patient Centered Clinical Trials conference. He continues the discussion by exploring the definition of patient centricity and issues that not only the FDA, but also the industry is facing.

As the discussion on patient centricity continues, industry personnel are attempting to further define the concept. John Whyte of the FDA sits down with us to explain his perspectives and experiences on the subject.

Vendor oversight in clinical trials is a subject not often discussed despite a disconnect in how it is carried out between multiple departments. Celeste Gonzalez of Boston Scientific shares her perspectives on the subject.

While many would like to see mHealth and wearable technologies incorporated in clinical trials, regulatory guidance has yet to be established for these innovations. However, some guidances do exist on the regulation and validation of mHealth use in consumer settings.

TransCelerate’s recently published article in a DIA publication covered issues management in clinical trial Quality Management Systems (QMS). Abbvie’s Susan Callery-D’Amico speaks to us about TransCelerate’s QMS Initiative and Issue Management.
As patient centricity continues to evolve, the differences in perspective between sponsors and the FDA over how to define this concept run parallel. With rising awareness will come the need for a definitive model that incorporates patient centricity in drug development.
The FDA’s launch of patient centric initiatives has led the industry to gear towards incorporating the patient’s voice in medical product development. Tom Krohn of Antidote discusses his company’s patient centered approach towards clinical trial matching by focusing on the patient.

As biopharma companies continue to explore and experience ways in which risk-based monitoring is implemented, the process of such can be misconstrued. Peter Schiemann elaborates on some of the current issues of RBM interpretation and implementation.