
In this interview, Dr. David Lee Scher adds his perspective on how digital health is changing in both healthcare and clinical trial settings.

In this interview, Dr. David Lee Scher adds his perspective on how digital health is changing in both healthcare and clinical trial settings.

The topic of clinical trial quality and compliance continues to evolve in clinical operations, based on discussions at ExL’s 9th Proactive GCP Compliance Conference.

In this interview, Kevin Hudziak will expand on Lilly’s initiatives to change the face of clinical trials through patient engagement and education initiatives.
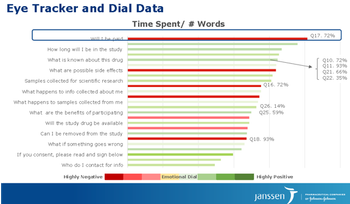
At ExL’s CROWN Congress, Cassandra Smith, Associate Director, Investigator & Patient Engagement at Janssen, discussed results from a study they conducted with patients on consent content modification.

This article will analyze industry clinical trial initiatives and investigate strategies on how to improve impact on people.

In this interview, Kevin Hudziak will expand on Lilly’s initiatives to change the face of clinical trials through patient engagement and education initiatives.

During the CROWN congress, there was discussion on CRO acquisition and consolidation, growing interest in the use of Blockchain technology in clinical trials, and new patient engagement methodologies.

In this interview, Jennifer Prichard, MD, Medical Monitor at Atlantic Research Group, and Hunter Walker, CTO at Atlantic Research Group will discuss challenges with existing medical monitoring processes, and how new technologies can help improve efficiency.

In this interview, Dr. Michelle Longmire, CEO of Medable, will discuss her perspective on the advancements of digital health in clinical trials.

Kristy Galante, Director Process and Infrastructure of External Alliances at Janssen, recently spoke about a novel vendor oversight model at ExL’s 8th Clinical Quality Oversight Forum, and will expand on the model in this interview.

In this interview, Julie Dietrich will provide more information regarding this Clinical Research Access & Information Exchange Initiative and how it is expected to benefit patients and the industry.

In this interview, Christa Polidori, Clinical Trial Disclosure Manager at Bristol-Myers Squibb and a leader for the TransCelerate Clinical Research Access and Information Exchange Initiative, will discuss the TransCelerate proposal in greater detail.

2017 has been an exciting year for the clinical trials industry on many fronts, driven by new methods, regulation, and technology, but what is to come in 2018?

In this interview, Austin Speier, VP of Emerging Technologies at Precision for Medicine, will elaborate on these draft guidance documents.

In this article, we will suggest an introspective method that can help clarify situational analysis and resolution.
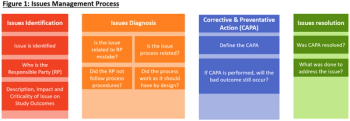
In this article, we will define a process on how to identify, diagnose, correct, and resolve issues in a QMS issues management repository.

Joe Pollarine, Head of GxP Systems Strategy Director at Janssen, recently spoke about CRO oversight models and will expand on these models in this interview.

The biopharmaceutical industry must establish customized approaches to managing Quality Management Systems within their clinical trials processes.

The Duke Margolis Center for Health Policy (DMCHP) recently collaborated with the FDA to release an mHealth action plan entitled "Mobilizing mHealth Innovation for Real-World Evidence Generation."
This article will dive into the details of FDA’s movement in mHealth, analyze FDA’s approach, and assess how this movement impacts the use of mHealth in clinical trial settings.

Austin Speier, VP of Emerging Technologies at Precision for Medicine, recently spoke about mHealth regulatory pathways at PanAgora’s Mobile Innovations Summit, and will elaborate further in this Q&A interview.
This article discusses the adoption of RBQM in academic settings and the lessons that were learned.

As the clinical trials industry starts to incorporate mHealth initiatives into their trials, study teams now have to determine the extent of their ICF consent forms during study deployment, and how to cover mHealth activities within these forms.

In this interview, we speak to Dr. Joanne Waldstreicher, Chief Medical Officer of J&J’s Office of the Chief Medical Officer (OCMO), to discuss its data sharing program via the Yale University Open Data Access (YODA) Project.

This Q&A discusses how the movement in making studies more patient-centric continues, and Pfizer is now implementing its own mHealth study in Lupus.
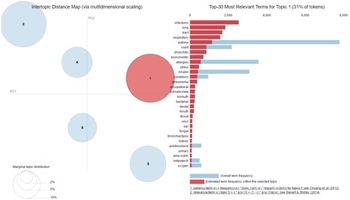
In this article, we will analyze themes from asthma patient conversations via HealthUnlocked, an online healthcare social network, and compare these themes to asthma clinical trial endpoints from large pharma studies.

Rob DiCicco, VP of Clinical Innovation and Digital Platforms at GSK, will discuss the TransCelerate protocol template initiative in this interview.
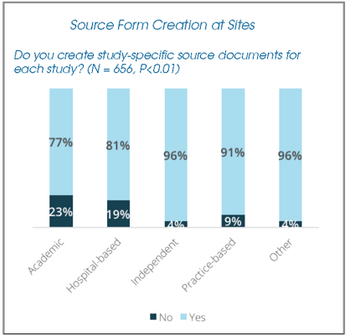
Tanya Bridges and Donna Benson, from two non-affiliated study sites, discuss the burdens of using paper source forms, and their impact on resources and trial execution at study sites.

We interviewed Pfizer exec, Dr. Jonathan Rowe, about how they are leveraging predictive models to manage study risk and quality.

We discuss the pilot siteless models in clinical trial patient centricity with Gabriel Vargas, an executive at Amgen.