
Dr. Peter St. Wecker, Director of Clinical Process Improvement & Innovation at Zogenix, offers his unique perspective on challenges and changes that sponsors and sites underwent during the COVID-19 pandemic.

Dr. Peter St. Wecker, Director of Clinical Process Improvement & Innovation at Zogenix, offers his unique perspective on challenges and changes that sponsors and sites underwent during the COVID-19 pandemic.
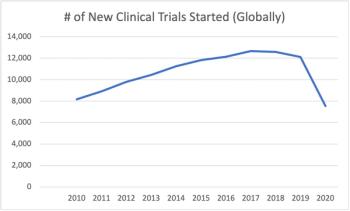
Though the industry was expected to boom with new studies—data shows a significant drop in the amount of new trials started in 2020

Pamela D. Garzone, Chief Medical Officer at Calibr, shares her perspectives on how investor decisions are shifting dramatically into biopharmaceuticals.

Jane Myles, Director of Decentralized Trials at Covance by LabCorp, and former Head, Operational Intelligence and Innovation at Roche, discusses her journey as a clinical trial participant.

Jessica Lee, SVP Clinical Operations and Global Integration at Inovio Pharmaceuticals discusses her perspective on the emergence of novel COVID variants and how they could impact results for sponsors currently running COVID-19 studies

Amy Davis, Sr. Director of Clinical Development Oncology at Eli Lilly, discusses her perspectives on diversity in clinical trials and the workplace.

NeuroRx CEO, Jonathan Javitt, and Head of Operations/CCO, Robert Besthof, discuss the logistics and trial progress of their COVID-19 drug.
What has changed in 2020? How will 2021 shape up? Let’s evaluate.

Ralph Passarella, CEO and Co-Founder, and Michael Lin, Executive Chairman and Co-Founder of Reify Health, discusses how workflow optimization technology adapts to address clinical trial recruitment issues.

Craig Serra, who works in Clinical Technology and Innovation at Novartis, discusses his perspectives on clinical innovation implementation during COVID.

A discussion of various topics amongst TMF experts from Questex’s 2020 Global TMF Summit.

Cue Biopharma has managed to persevere through the COVID pandemic by employing a variety of strategies. President and Chief Scientific Officer, Anish Suri, and Ken Pienta, acting Chief Medical Officer discuss their experiences.

A discussion of how sponsors, CROs, and sites are adapting their operational models to maintain patient safety, data quality, and integrity in the COVID-19 pandemic.

Both quality personnel and the FDA have predicted some of the issues with pandemic operations, but much is still unknown on the impact these rapidly implemented pandemic processes.

Disa Lee Choun, Head of Innovation at UCB, discusses how they have fostered a culture of innovation, and how the investment in piloting has benefitted clinical operational implementation during the COVID-19 pandemic.

Sudarshan Hebbar, CMO of CalciMedica, reveals how the FDA, ethics committees, and safety consortia have responded to helping to advance the application of novel therapeutics towards COVID.

Shawn Singh, Chief Executive Officer of VistaGen Therapeutics, discusses the company's ongoing efforts to treat and help manage the anxiety symptoms related to the triggers and anxiety-related disorders COVID-19 provokes, and the FDA’s consensus on the design of their pivotal Phase III study of PH94B.

Rasmus Hogreffe, former Head of Virtual Trials at LEO Innovation Lab, and current VP of Decentralized Trial Innovation at Medable, and Morten Kirkegaard, Head of Clinical Operations and Co-founder at REDO-neurosystems, discuss their experiences with decentralized trials.

Dr. Michelle Longmire, Chief Executive Officer and founder of Medable, sits down with Moe Alsumidaie to discuss how the decentralized model will transform how trials are executed for years to come.

Sangamo executives, Melita Sun Jung and Amy Pooler, discuss the future of genomic medicines for autism spectrum disorder.

Firma Clinical Research CEO Michael Woods shares why COVID-19 may force greater efficiency for trial designs and greater access for trial participants.

Rasmus Hogreffe, former Head of Virtual Trials at LEO Innovation Lab, and current VP of Decentralized Trial Innovation at Medable, and Morten Kirkegaard, Head of Clinical Operations and Co-founder at REDO-neurosystems, discuss their experiences with decentralized trials.

A look at the evolution of clinical trial recruitment, challenges the industry still faces, and how it can use automation to enhance enrollment efforts.

CEO of Firma Clinical Research, Michael Woods, shares why COVID-19 may force greater efficiency for trial designs and greater access for trial participants.

G1 Chief Operating Officer, Terry Murdock, discusses how the company is navigating the evolving COVID-19 environment.

JoAnne Schaberick, Co-CEO of Pro-ficiency, discusses how their simulation training platform is helping provide remote clinical trial site training to sponsors and study sites.

Lori McDermott, VP, Clinical Development & Regulatory Affairs at Heat Biologics, discusses their company's approach towards developing a COVID therapy.

Dr. Rolland Carlson, CEO of Immunexpress, discusses how the company overcame the challenges of implementing sepsis clinical trials.

A summarization of the topics discussed at ExL’s 10th Annual Clinical Quality Oversight Forum.

Tom Rhodes, CEO of Spencer Health Solutions, discusses his experiences on how the biopharmaceutical industry has been impacted by COVID, and what some are doing to succeed.