
The disengagement of major drug firms is described as "a type of market failure" in a briefing paper published last month by the UK think-tank Chatham House.

The disengagement of major drug firms is described as "a type of market failure" in a briefing paper published last month by the UK think-tank Chatham House.
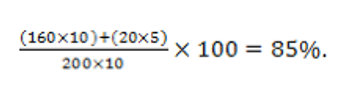
Quality metrics are hard to define and measure, but are crucially important.

The final version of the EMA Reflection Paper on Risk Based Quality Management in Clinical Trials is now available.

Innovative uses of social media and other new information technology has become increasingly important in improving clinical research operations and sponsor communications with sites, investigators and vendors.

A new call for proposals is going to appear in December from the Innovative Medicines Initiative, the European Union partnership with the pharmaceutical industry.

Global action and collaboration is needed to tackle the threat of antibiotic-resistant diseases.

Earlier this month, we hosted a webinar supported by MAPI on the topic of Risk-based Monitoring Tools for Application.

The bacterial meningitis outbreak at Princeton University in recent weeks has raised questions about why there is no vaccine in US to prevent this deadly disease, when such a therapy is approved in Europe and Australia.

The annual worldwide antibiotic awareness event--celebrated in Europe on November 15--generated some alarming statistics, some reassuring statements from official sources, and some expressions of urgency from drug developers.

This webcast is free and the expert speakers from Price Waterhouse Coopers, Industry Standard Research and SAS will be discussing their own research and experiences on this topic.
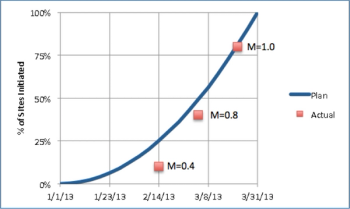
We'll start our monthly metrics blog with a basic but crucial MCC Clinical Trial Performance metric: on-time site initiation.

Sample banking for future clinical research provides the pharmaceutical industry with new opportunities to obtain biological sample collections.

If you are in clinical research, you may read a headline such as "Thomson Reuters Previews Game-Changing New Solutions for Insights into Clinical Trials" with passing curiosity... along the lines of "what do they have to do with clinical trials?"

Cloud computing is changing enterprise across all industries, and the recent success of Veeva's IPO is further evidence that the life science industry is no exception.

Given FDA's new guidance on risk-based monitoring (RBM), there has been a lot of interest on identifying critical data points, drafting RBM plans, and executing RBM.

Compromise is going to play a key role as the clinical trial rules update debate rages on.

April might be - as T S Eliot suggested - the cruellest month, but for pharmaceuticals in Europe, this October is certainly a contender to win an award as the busiest month.

Over the past several years, we have been seeing an influx of significant changes in the clinical trials industry ranging from changes in the way we approach clinical trials, and massive growth in new technologies.

Over the past few months the majority of the news coming out of the pharmaceutical industry has been a refrain of slashing R&D spending as a result of increasingly competitive drug development.

That's one of our key aphorisms here at the Metrics Champion Consortium (MCC). We passionately believe that measurement can effect change, and we?ve spent the last eight years working with our members to define the best metrics for tracking performance and improvement in the industry.

Validation - a word we use a lot in this industry. For Triumph Consultancy Services, it is usually a term which we associate with systems development, but on this occasion I'm excited to say I'm talking about validation of our new business division and its ideas.

Last year, I attended the Sponsor/CRO Business Process Integration conference at this same time in New Brunswick, and it was one of my favorites. This year, it's in Raleigh NC.

Europe moved one step closer to a passport for physicians in early October.

We've been working with our friends at Medidata on a series of online edicational tools on the topic of integrating IRT and EDC.

With so many sponsors and CROs focused on finding the right technology solution for implementing risk based monitoring, a key element often gets overlooked--the risk assessment.

Due to increasing costs and lacking productivity of the existing clinical trial operational model, there has been a lot of awareness around ?Future Clinical Trials,? and some sponsors are seeming to transform pharmacies into study sites.

How Punit Dhillon, CEO, OncoSec Medical, hopes to leverage online communities to design better oncology trials.

Officials from the Food and Drug Administration and the National Institutes of Health were scheduled to explain developments in clinical trial registration and transparency at the Drug Information Association?s conference on Clinical Trial Disclosure in Bethesda, Md. this week.
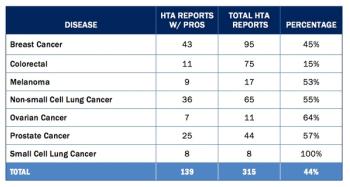
In a recent piece published in the New England Journal of Medicine, oncologist Dr. Ethan Basch talks about his perspective that patient-reported outcomes (PROs) are not widely used in the development process for cancer drugs because they are not often measured in clinical trials.

Will the clinical trials community assemble their arguments, and will they do it in time?