
Key differences and gaps in requirements for testing and documenting product similarity have emerged among the European Union, the US and other regions.

Key differences and gaps in requirements for testing and documenting product similarity have emerged among the European Union, the US and other regions.

What can be gleaned about the direction that health policy will take for the next five years under the new European Commission, scheduled to take office at the beginning of November?

President and Co-founder of Canadian CRO Scimega Research, Denise Deakin, addresses last week's article published in the Toronto Star

Inevitably, the conversation consistently comes back to a simple philosophical debate?should software solutions be configurable or customizable?

There are various estimates in the public domain that applying big data strategies could potentially generate up to $100 billion in value annually across the US healthcare systems.

The rush is on to develop new therapies and preventives to combat the lethal Ebola outbreak.

It's no secret that there is increased clinical focus now on the prevention and early detection of Alzheimer's.

Eye for Pharma hosted its first Patient Centered Clinical Trials symposium in Boston.
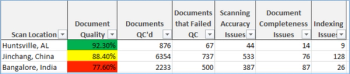
Even though sponsors and CROs are working to create more and more documents as electronic originals, scanned paper still comprises a large portion of any given eTMF.

The new Clinical Trials Regulation introduces an overhaul of the existing regulation of clinical trials for medicinal products in the EU.

It is a tragic truism to remark that the merits of the pharma sector are most sharply perceived only at times of deep human suffering.

The health sciences industry received some important and encouraging news from the US Food and Drug Administration in late June...

Last August, Quintiles announced its intention to purchase Novella Clinical, specifically for its focus on small and mid-sized oncology biopharma clients, as well as medical device and diagnostics companies.

While at the 2014 NYBIO conference, Dr. Sam Waksal from Kadmon had the opportunity to address the audience regarding the state of the biopharmaceutical industry.

Rare disease research offers a lot for people in the world of clinical trials and drug development to think about.

As Europe departs for its annual holiday the question remains unresolved as to what to expect from the new European Parliament in terms of the principal interest of readers of Applied Clinical Trials.

In mid-July, the SAS Press Program released a new book titled Risk-Based Monitoring and Fraud Detection in Clinical Trials Using JMP and SAS.
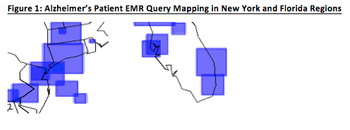
The topic of subject enrollment has been evaluated from many different perspectives.

After months of speculation about prospects for biosimilar development in the US, Novartis announced July 24 that FDA has accepted Sandoz' biologics license application ...

It's a given that events will happen during the course of the trial that will require not just new documents, but new sets of documents.

This article will discuss FDA's post-marketing surveillance programs, and how biopharmaceutical sponsors can work with the FDA to identify unreported Adverse Events.
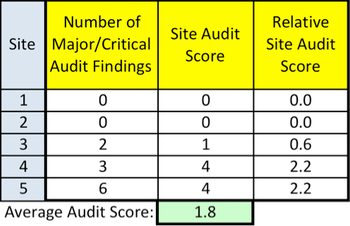
MCC has just published an executive summary of its Risk Based Monitoring Usage Survey.

The European Union's struggle to bring its data protection rules into the 21st century continues...

One event which you may have missed at DIA was the launch of a new suite of eClinical solutions by Techorizon.

A potential treatment for sickle cell disease has come through early stage development due to support from a collaborative partnership established by NCATS at the NIH.

Dr. Paul Wicks, head of innovation at PatientsLikeMe, detailed for Applied Clinical Trials, the three services the company announced at DIA.

Recruiting patients remains one of the most difficult challenges clinical trial sponsors face.

Key to accelerating the discovery and development of new medical therapies is to improve the clinical research process.

The pharmaceutical industry continues to outsource more clinical trials to contract research organizations each year...

The European Union has managed to snatch defeat from the jaws of opportunity once more!