
Trulicity® (dulaglutide), approved by the EMA in November 2014 for the treatment of Type 2 diabetes in adults, is the first diabetes drug to have a Patient Reported Outcome.

Trulicity® (dulaglutide), approved by the EMA in November 2014 for the treatment of Type 2 diabetes in adults, is the first diabetes drug to have a Patient Reported Outcome.

eSource is not a new term. But eSource does seem to be getting a lot of attention lately.

ResearchKit is Apple’s foray into clinical research based on the backbone of its HealthKit, released in June 2014, which offered the ability for health and fitness apps to communicate with each other. mHealth News reported Apple CEO Tim Cook said, “As we worked on HealthKit, we came across an even broader impact that iPhone could make, and that is on medical research.”
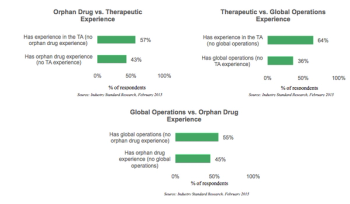
Despite the substantial difficulties associated with orphan drug trial recruitment, this segment of the drug development market is booming.
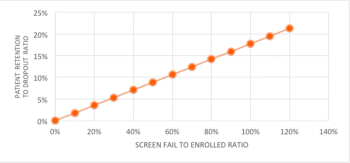
Clinical trial patient retention and dropout continues to be an issue amongst biopharmaceutical sponsors, as patient dropouts minimize the statistical power of clinical trial data, requiring study teams to enroll additional patients.

Over the years, clinical study management has become more fragmented. There are more organizations participating in a study, each bringing their own experiences, interpretations of requirements including how quality is defined. How is it possible that requirements and quality be interpreted so differently?

Patient focused drug development (PFDD) is moving into the mainstream, promising to alter the conduct of clinical trials and FDA regulatory policies.

Managing a successful Phase III global oncology trial presents one of the most complex challenges drug companies face within the development cycle.

Historically, some longstanding challenges have hindered efforts to integrate patient data from EMRs (Electronic Medical Record systems) with EDC (Electronic Data Capture) systems.

The European Medicines Agency's public consultation on yet another aspect of transparency on clinical trials data has elicited further critical comment about excessive official secrecy.

The potential for mHealth to positively impact clinical trials is extraordinary, but as we’re in the early days, there are far more questions than answers.
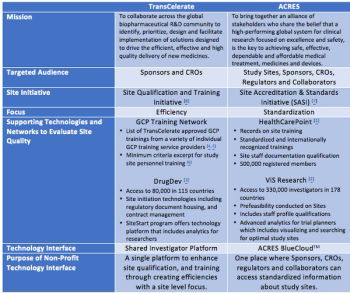
Over the past several years, there has been a rise in nonprofit organizations that focus on addressing the challenges in clinical research.

ERT’s announcement of its intention to acquire PHT makes more than sense. And when speaking with the companies’ CEOs, Jim Corrigan and Philip Lee respectively, the potential for success is greater than any challenges the current clinical trials market could throw their way.

It seems only yesterday that the future for European pharma was personalized medicine. The European Union first gave the term official status in a formal paper in 2008, entitled a Renewed Vision for the Pharmaceutical Sector, in which the Commission included a section on ‘Towards more personalized medicines’.

One of the primal questions clinical trialists engaged in clinical research and development always face is do we “keep or kill” a drug based on the data in hand.

Often overlooked in early evaluation of RBM are the tools and technologies required for successful implementation. Without the right tools to track and prioritize the shift from traditional monitoring to RBM, successful implementation is doubtful, posing detrimental consequences not only for the monitors, but also the overall trial.

That's because the discussion shifted away from one focused on changing monitoring methods and doing reduced source document verification (SDV) to 'intelligent monitoring.'

Patient adherence is an emerging topic and a matter of concern in clinical research. It applies to several different clinical trial facets.
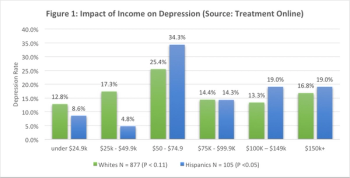
What leads to Patient dropout? Read more here.

At a time of the year when love is in the air, one thing is for sure: there is not a lot of love from the human research community on NIH’s Policy on the Use of a Single IRB for Multi-Site Research.

The ongoing battle over drug reimbursement and pricing has raised questions about whether the pharmaceutical industry can continue to rely on high U.S. revenues to fund biopharmaceutical R&D. Payers and insurers have become more aggressive in demanding clear evidence of value for high-priced medicines and are rejecting old models for coverage and reimbursement.

This week, I attended CBI’s Expanded Access Programs conference in Philadelphia.

While such discussions have advanced in Europe, two key omissions from the dialogue may limit any real changes.

The idea is great: when an investigator submits a grant to any NIH institute, if the study will involve more that one performance site, the grant must include a plan to rely on one single IRB.

One way to reduce off-label prescribing of treatments for cancer and other conditions is for manufacturers to test additional indications and file supplemental applications to add those uses to approved labeling

The concept of risk-based monitoring (RbM) is evolving, as nonprofit organizations continue to collaborate with the biopharmaceutical/medical device industry to investigate, pilot and implement RbM practices.

Efforts are escalating to encourage sponsors, research institutions and clinical investigators to accept oversight for multi-center studies by central Institutional Review Boards (IRBs).

How the European Union is impacting current Pharmaceutical trends.

Life sciences organizations run a challenging gauntlet when it comes to clinical development.

Fundamental weaknesses of modern clinical development can be resolved through recent statistical advances.