
My recent blog has been overtaken by history, with the departure of Guido Rasi from his post of Executive Director of the European Medicines Agency

My recent blog has been overtaken by history, with the departure of Guido Rasi from his post of Executive Director of the European Medicines Agency

The image acquisition and analysis components of R&D and clinical trials can be quite complex...

Designing protocols to maintain a trial's validity while enabling seriously ill and informed participants to receive both SOC and investigational products will help to improve patient participation in oncology clinical trials.

Growing insistence on making healthcare more patient-centered is generating a new level of interest in helping patients to meet the expectations the new orthodoxy is creating.

The 2014 mid-term elections handed over control of the Senate to Republicans and boosted the GOP majority in the House.

Here is a summary of what analysts are saying about LabCorp's purchase of Covance.

So now we know who is to do what on health issues in the new European Commission. Or do we?

We hear it all the time these days: research processes have to undergo transformative changes in order for research organizations to thrive?or even survive.
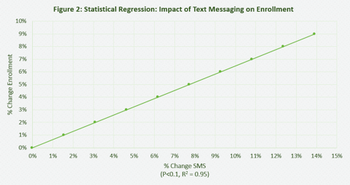
There is a lot of new research that evaluates the impact of Short Messaging System (SMS, Text Messages) in healthcare settings.

Central laboratory analysis of samples collected by the investigational sites is an important source of safety and efficacy endpoint data.

Leading Senators are proposing legislation to add Ebola to the list of diseases eligible for priority review vouchers from the FDA.

This month, the New England Journal of Medicine published the results of data sharing from pharmaceutical companies to researchers.

What's been a commonplace technology for larger organizations is now becoming more accessible economically to smaller enterprises. But how to choose?
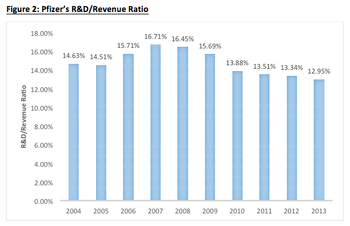
Is Pfizer prime for acquiring another biopharmaceutical company?

Issues being discussed with the Ebola vaccine sound like issues discussed with clinical trials in general. What can we learn?
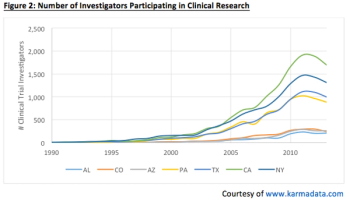
There are a number of indicators that suggest that US clinical trial growth is far from plateauing, and demand for clinical research investigators will continue to rise with this growing trend.

Spoiler alert: most IRT systems are not meeting user expectations in terms of eClinical integration.

Patients there respect and trust their doctors and do what he or she tells them to do...like participate in a clinical trial

Applied Clinical Trials wanted to know if Tess had any thoughts or recommendations around clinical trials after being a participant.
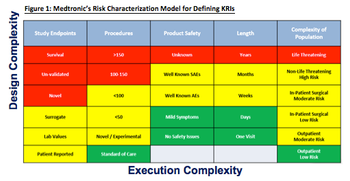
The industry has tried to establish some consistency by standardizing RBM methodologies.

Felix A. Khin-Maung-Gyi, executive chairman and founder of Chesapeake Research Review LLC and an ethicist whose field was human research, will be remembered.

To date, Quintiles has been the only CRO with representations at events hosted by the House of Representatives Energy & Commerce Subcommittee on Health's 21st Century Cures initiative.

To understand just how far mobile and digital technology can truly influence progress in global healthcare, we first need to form the foundation of the discussion with a few rudimentary facts.

Bio-pharmaceutical companies are continually tasked with gathering more safety information and responding to requirements more quickly. Recent examples of significant changes in reporting requirements include 2010/84/EU and Regulation Number 1235/2010 on pharmacovigilance, and the 2012 European Medicines Agency (EMA) new guidelines on good pharmacovigilance practices.

The federal Open Payments program went live Sept. 30.

Reshuffling to heavily impact life sciences policy in Europe.

Two main themes arose from the ePatient Connections Summit: online patient engagement and patient centricity.

Effective leadership is needed in a trial leader, but what exactly does that entail?
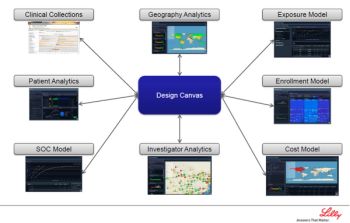
Thomas Krohn, Director of Clinical Open Innovation at Eli Lilly, showcased a breakthrough study design platform and process that study teams are currently utilizing.
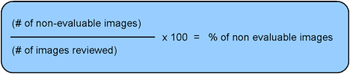
Medical imaging is an important source of subject eligibility, drug efficacy, and safety data for many clinical trials.