
Functional service provider arrangements, or FSPs, are evolving beyond the clinical data management tasks they were designed to streamline.

Functional service provider arrangements, or FSPs, are evolving beyond the clinical data management tasks they were designed to streamline.

Health technology assessment is a field of analysis that evaluates the medical, social, ethical, and economic implications of development, diffusion, and use of health technology.

Is the FDA doing enough to incorporate the patient perspective in the drug review process?

Regulatory authorities routinely inspect clinical trials, sites, sponsors, investigators, and other relevant parties to ensure compliance with good clinical practice guidelines.

Objective internal monitoring can help clinical trial sponsors transform a regulatory mandate into a valuable quality improvement tool.

An article that sets the stage for why improvements in the contract negotiations with sites has and needed to improve for the past two years.

Patient consent is important to stay on top of for many reasons.

PCORI underscored existing trends toward increasingly individualized and personalized medical care...

The advent of Accountable Care Organizations is causing providers to rethink their legacy financial models as they seek to effectively quantify and understand the clinical or financial experience of their patients.

DIA held its 2014 annual meeting in San Diego and attracted thousands of attendees from around the globe.

As the new European Parliament assembles for the start of its five-year term on July 1, many familiar faces will be missing.

The clinical trials world is starting to adapt computer-aided design.

Amid all the heated discussions of transparency and the insistence on ever wider disclosure, an intriguing contrast is provided by a document signed by health ministers on June 20.

Pharmacovigilance is the next frontier for outsourcing in the pharmaceutical and biologics industry.
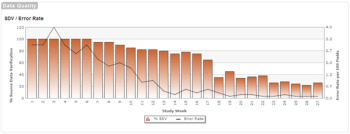
This is the third post in a three-part series on quality implications of RBM.

We got the chance to speak with Nancy Dreyer, chief of scientific affairs and senior VP at Quintiles Outcome about the current tide for comparative effectiveness research

Historical information and analytical foundations for RBM are important, but, in my view, RBM is about proactively managing each individual trial to quality and efficiency goals.

Companies that don?t collect pharmacokinetic data during Phase II and Phase III clinical trials are missing major dividends.

But the re-brand of PRA Health Sciences from PRA International strikes a different tone than most.
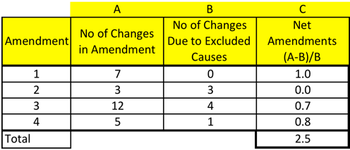
This month, let's look at a quality metric that's critical to on-time, on-budget performance: protocol amendments.

Risk-based monitoring provides an opportunity to make the work of CRAs more interesting.

Patient enrollment for clinical trials is not a numbers game.

An effective clinical trial is one of the most crucial stages in the creation of a new drug.

A series of pieces examining significant past peer-reviews articles.

A well-informed update on how the problems in the Ukraine are impacting clinical trials in the region.

While at the 2014 New York BIO conference, FDA Commissioner, Dr. Margaret Hamburg, had the opportunity to address pressing issues affecting the biopharmaceutical industry and the FDA.

Patients 65 years of age and over are underrepresented in clinical trials, even though in many therapeutic categories they are the primary drug consumers.

We compiled a list of how sponsors offer access to patient level clinical data requests from researchers.

While at the 2014 New York BIO Conference, I had the opportunity to interview Nathan Tinker, Executive Director at the New York Biotechnology Association, about challenges in early phase development.

Claims of success for pharmacovigilance are under question, but hopes are higher for new clinical trials rules.