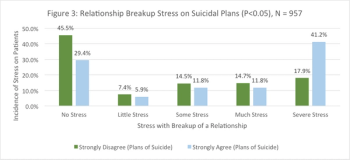
This article will analyze factors that affect suicidal ideation from a clinical psychiatric pilot study.

This article will analyze factors that affect suicidal ideation from a clinical psychiatric pilot study.

Investigators and sponsors of clinical trials will have to make more detailed data available following study completion, according to a new report from an Institute of Medicine expert panel.

Combing for clinical trials patients and the best way to make the match between trial and patient is a multifaceted task.

Professor Sirpa Leppä pointed to the increasing challenge of inducing trial sponsors to select Finland in recent article.

The main surprise at the Jan. 7, 2015 meeting of FDA’s Oncologic Drugs Advisory Committee was the panel’s strong support for a drug developed under a very different model from most cancer therapies.

The European Union's extensive transatlantic links have reached a further level of maturity with the completion at the end of 2014 of exchanges between the European Medicines Agency and Health Canada.

The entry into force of the new EU clinical trials regulation last June is really little more than a virtual event.

Over a year ago, Claravant started the research and vetting required to “handicap” the risk of a drug being approved by FDA, and ultimately becoming commercialized in the form of a ratings system.

It's official. Europe is sick-and much of the sickness derives from its economic problems.

Patients in Europe have been increasingly vocal in their demands for engagement in drug development and regulatory processes-and they have been increasingly effective.
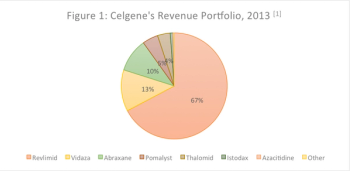
This article will analyze the impact of Revlimid on a different outcome: multiple myeloma mortality rates in the US.
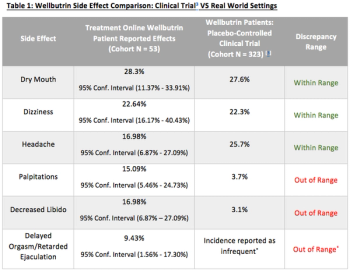
This article will evaluate some concerns regarding psychiatric clinical trial design, and will analyze post-marketing patient reported outcomes data on the antidepressant, Bupropion HCL (Wellbutrin), from Treatment Online, an online psychiatric treatment platform.

FDA set several milestones in approving more new, important drugs and biologics in 2014.

Patient access to critical experimental medicines continues to grab public attention, as states enact “Right-to-Try” laws and Congress eyes establishing a national policy to provide not-yet-approved therapies to terminally ill patients.

FDA has scheduled a public meeting in early January to assess and weigh the data on the first US application for a biosimilar therapy.

After nearly five years of argument, the European Union's proposal to update its transparency directive for medicines has been dropped.

Building on more than a decade of initiatives to spur pediatric labeling on drugs and biologics, regulatory authorities are bolstering incentives for sponsors to develop more information faster on the use of medicines in children.

While the issue of adverse drug reaction (ADR) reporting is apparent in hospital systems, a deeper and uninvestigated problem resides within the process of ADRs: the patient.

In the coming year, we expect to see sponsors focused on making better use of their data investments that are now electronically available through EDC and other systems.

It’s clear that the value clinical trials bring to the health and well being of our existence is being recognized and expectations from the public are high.

The development and approval of new vaccines and antivirals to contain and treat the Ebola virus outbreak has become a top priority for the federal government and Congress.

PCORI is launching a $50 million program to fund research on the comparative effectiveness of various approaches for diagnosing and treating hepatitis C.

The conclusions on vaccination that European Union health ministers adopted on December 1 contain several sound thoughts.

Clinical trials need to become a part of the treatment continuum, not separated from it as it is now.

For each clinical trial, a clinical trial agreement and budget are negotiated between the investigator and the sponsoring company so that the costs of carrying out the trial are reimbursed.
There is a lot of discussion and awareness on the topic of patient centricity, as many experts are starting to discuss how the concept can improve clinical trials.

In a health policy world where competition for profile is tough, a vacuum at the top is a serious disadvantage.

The two-year-old initiative to accelerate the development and approval of highly effective drugs and biologics has enabled a number of important new medicines to reach patients sooner, according to Janet Woodcock.

US researchers and regulators continue to support the use of randomized clinical trials to test potential treatments and vaccines to combat the Ebola virus...

I recently visited the Caribbean isles, and on my way at the airport, I noticed prominent CDC disclaimers about an equatorial viral disease: Chikungunya.