Applied Clinical Trials
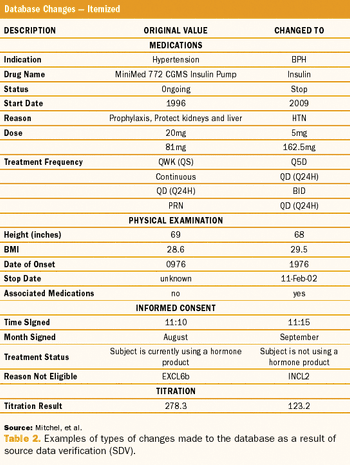
Study results using a quality-by-design method, risk-based monitoring, and real-time direct data entry.

Applied Clinical Trials
Study results using a quality-by-design method, risk-based monitoring, and real-time direct data entry.


Applied Clinical Trials
Clinical trial optimization through adaptation in several key areas was a prevailing message at the recent Partnerships Conference.

Applied Clinical Trials
Congressional leaders have launched a major initiative to revise laws and regulatory policies to speed new cures to patients.

Applied Clinical Trials
Redefining this paradigm may help dispel current misperceptions around RBM and better deliver on the promise of this growing approach.

Applied Clinical Trials
Science exhibit will give children and young adults a rare look inside clinical trial patient experience.

Applied Clinical Trials
Claims of success for pharmacovigilance are under question, but hopes are higher for new clinical trials rules.

Applied Clinical Trials
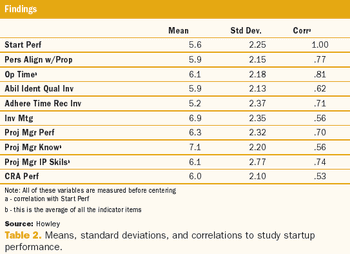
A two-phase statistical analysis identifies the key performance drivers in clinical trial startup.

Applied Clinical Trials
How iterative analysis of subject recruitment data can optimize outcomes during ongoing enrollment.
Applied Clinical Trials
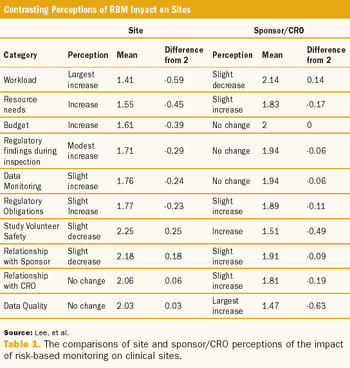
Sponsor-site alignment on risk-based approaches is critical if clinical trial standards are to reach new levels.

Applied Clinical Trials
CRO/Sponsor: High Performing Study Startups Sites: RBM Challenges, Opportunities Trial Design: Three-pronged Monitoring Approach Study Recruitment: Enhancing Trial Enrollment Also in this issue: New EU Drug Legislation The Patient Experience Rebranding RBM