
News





SUBJECT RECRUITMENT : Integrated Team - Sponsors, CROs, and Sites Patient Tracking Services Can Help Reduce Lost to Follow-Up TRIAL DESIGN : Pediatric Trials - Errors in Informed Consent Also in this issue : Common Rule Revision, EU?s Health Policy and Clinical Trials, CROs Going Private, Research Clusters
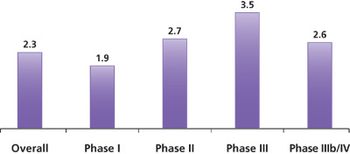
A recent study conducted by the Tufts Center for the Study of Drug Development (Tufts CSDD) found that more than half of all protocols require one or more amendments.

The EMA is moving its offices to Canary Wharf Estate.

Companies such as ExcoInTouch, Pfizer, and others are turning to mobile technology.

Industry news focusing on the people and organizations who work in the clinical trials profession.

The New England Journal of Medicine reveals list of biosimilar guidelines.



















2011 Directory & Buyers Guide Services & Products Company Index