
News
Advertisement


Advertisement



17th Annual Special Resource Issue CDISC Glossary US Departments and Offices European Regulatory Agencies Update Comprehensive 2012 Calendar
Advertisement







Challenge to coverage mandate would undermine efforts to achieve universal coverage.






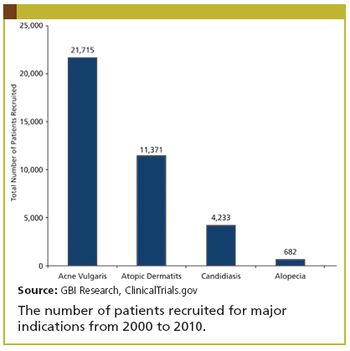
Updates in dermatology trials.

Industry news focusing on the people and organizations who work in the clinical trials profession.

Perceptive Informatics is providing the core clinical trial technology applications to Pfizer's REMOTE trial.

Biobank research has a valuable role to play in R&D, but handling the consent process is rarely straightforward.
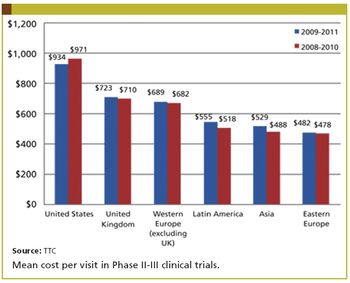
Site per visit increases have been smaller in recent years.

Improving Clinical Operations with Digital Signatures




Advertisement
Advertisement
Trending on Applied Clinical Trials Online
1
SCOPE Summit 2026 Keynote Panel: Is Radical Acceleration in Clinical Research Possible?
2
SCOPE Summit 2026 Panel Discussion: Diversity in Clinical Trials—What’s Working, What’s Next
3
SCOPE Summit 2026: Reducing Patient Burden Is the Foundation of Wearable Success in Oncology
4
SCOPE Summit 2026: Elevating Patient Experience in Clinical Operations
5