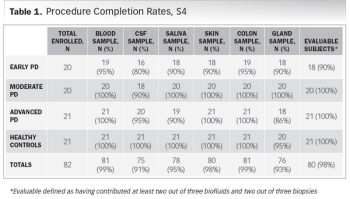
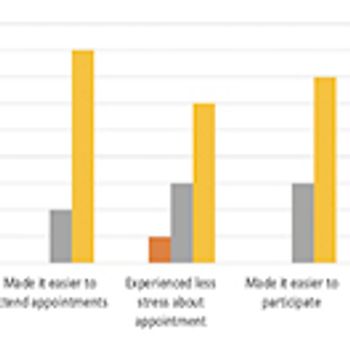
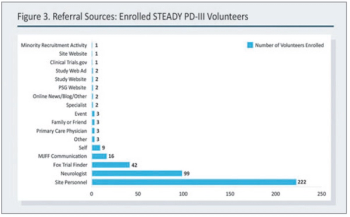
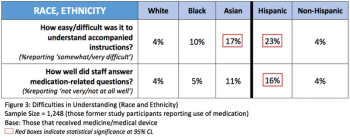
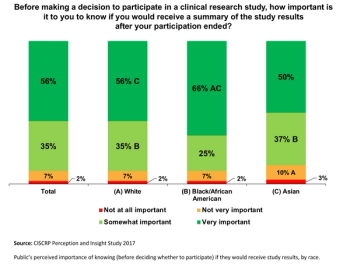
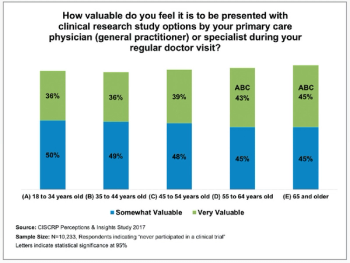
The pandemic has thus far disproportionally impacted minority populations, and our ongoing failure to adequately represent all patients regardless of demographic background has never been more important to remedy than it is today, writes ACRP Workforce Innovation Officer Beth Harper.