
Real World Evidence
Latest News
Latest Videos
More News

Craig Coleman, PharmD, Professor at the University of Connecticut, and the Observational Study in Cancer-Associated Thrombosis for Rivaroxaban (OSCAR) study's principal investigator discusses the ins and outs of the study.

Addressing Operational and Technical Challenges in Home-use Point-of-Care Device Development and Deployment
Webinar Date/Time: Mon, Feb 13, 2023 10:00 AM EST

Webinar Date/Time: Tuesday, December 13, 2022 at 10am ET | 3pm GMT | 4pm CET

Survey among readers evaluates the trajectory of RWD and how it can be more widely adopted.

Addressing sources of tension on differentiating types of data.

By integrating real-world data more deeply into the process of clinical research, life sciences stakeholders can open up new possibilities for therapeutic development and the evidence-based treatment of hematologic cancers.

RWE can augment, extend, or enrich the findings from clinical trials to provide valuable evidence to support the development programs for product approvals.

C.K. Wang, MD, senior medical director at COTA Inc., discusses the impact of RWD and RWE in clinical oncology.

A look at the scope of the agency’s draft framework for evaluating the use of RWE to support new drug approvals and the implications for sponsors.
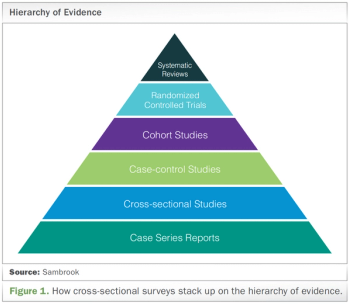
With outcomes from “real life” a critical compliment to clinical trial data, the importance of involving an epidemiologist at study inception is explored.

Karen Hill and Heather Fitzpatrick Medlin discuss the recent advancements and challenges in cardiovascular and metabolic studies, as well as strategies to address current obstacles and advance the industry further.

Study examines the growing integration of real-world data and evidence and the remaining roadblocks to adoption.
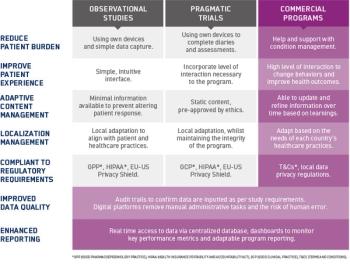
In this three-part blog series, we look at how electronic platforms can support and enhance data capture in several types of RWD programs: observational studies, pragmatic trials, and commercial programs.
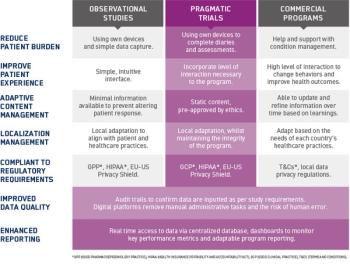
In this three-part blog series, we look at how electronic platforms can support and enhance data capture in several types of RWD programs: observational studies, pragmatic trials, and commercial programs.
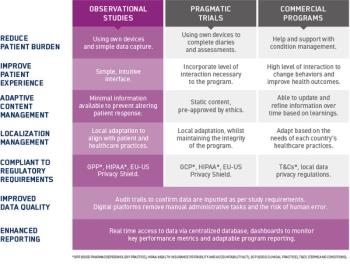
In this three-part blog series, we look at how electronic platforms can support and enhance data capture in several types of RWD programs: observational studies, pragmatic trials, and commercial programs.

How pragmatic clinical trials increase the robustness of real world studies at a fraction of the cost of classical randomized controlled clinical trials.

The early implementation of post authorization safety studies (PASS) will translate to efficiencies in pre-appoval research. These benefits allow for faster approval and wider patient access to potentially life saving therapies.

Forging new partnerships focused on the safety of long-acting opiates is important, as public health questions surrounding drug abuse and addiction have increased, along with regulations governing these products once they reach the market.
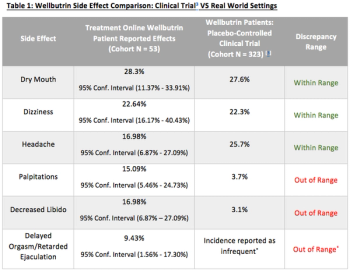
This article will evaluate some concerns regarding psychiatric clinical trial design, and will analyze post-marketing patient reported outcomes data on the antidepressant, Bupropion HCL (Wellbutrin), from Treatment Online, an online psychiatric treatment platform.








