
16th Annual Special Resource Issue CDISC Glossaries Professional Societies and Associations Training and Education Comprehensive 2011 Calendar

16th Annual Special Resource Issue CDISC Glossaries Professional Societies and Associations Training and Education Comprehensive 2011 Calendar





REMS Operational Challenges: Considerations in REMS Development and Long-term Management











SAS - A Marketer?s Guide to Analytics

SAS - Turning Customer Data into Analytical Marketing Fuel




SURVEY : 2010 Salary & Satisfaction Survey CRO/SPONSOR : Mergers and Acquisitions Prevalent in Pharma LABS : Discordance Between BICR Readers Also in this issue : $500 Million a Year Earmarked for Disease Research, EMA Addresses Possible Conflicts of Interest, Investigative Sites in Financial Trouble, Suicide Risk Monitoring
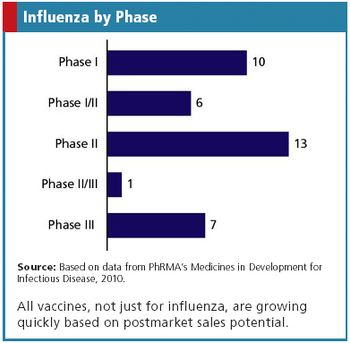
Updates on influenza vaccines.
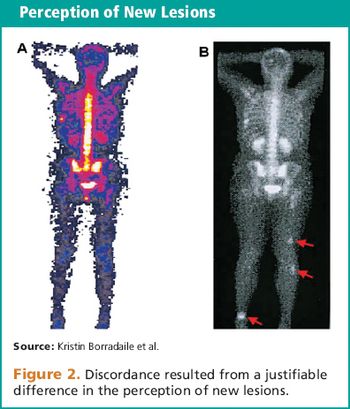
Understanding the causes and implementing processes to mitigate preventable sources of discordance.

Centralized approach for Phase III studies enhances the quality and integrity of collected data.
How does ECG reading in clinical research compare to a healthcare interpretation?
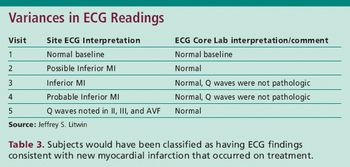
Understanding the critical elements and challenges of cardiac safety trials.