
News






Partnerships in Clinical Trials

TRIAL DESIGN : Rare Disease Research and Patient Registries Safety: Drug Exposures in Expectant Mothers SUBJECT RECRUITMENT : How to Raise Accrual Rates in Cancer Trials Also in this issue : FDA?s Sentinel System, Clinical Trials Directive, One Hundred Percent Source Data Verification, Understanding Medication Non-Adherence

Cover

Industry news focusing on the people and organizations who work in the clinical trials profession.

Chinese pharmaceutical company forms alliance with American CRO.

Direction from regulatory agencies would help eradicate wasteful 100 percent source data verification.
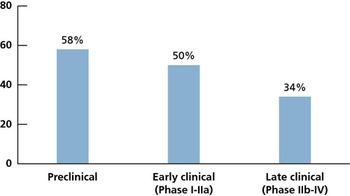
A 2010 survey conducted by the Tufts Center for the Study of Drug Development (Tufts CSDD) finds that sponsor companies are allocating resources, modifying organizational structures, and increasing investment in the development of personalized medicines (i.e., tailoring of medical treatment and healthcare delivery based on individual patient characteristics including genetic, molecular, and imaging).

Cover

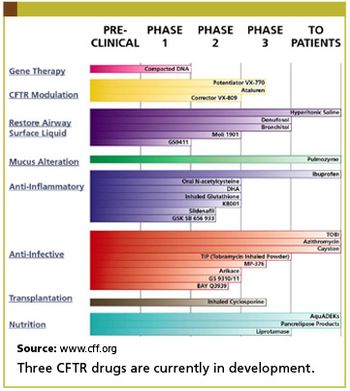
News from the Orphan Drug workshop.

The recent shutdown of the R&D facility should signify a warning to the British science community.













