
News






















GLOBAL TRIALS : Trials in the Middle East and North Africa Historical Overview: United Kingdom Trials KKS Network Increases Output in Germany Also in this issue : Presidential Panel Considers Tighter Rules, European Union Provides Guidance, Natural Language Processing, Reducing Costs Through Technology

Industry news focusing on the people and organizations who work in the clinical trials profession.

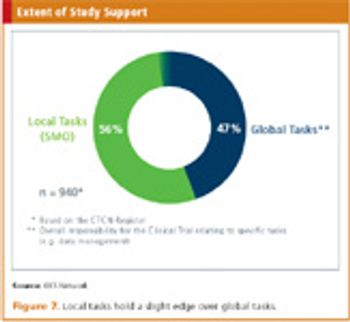
The founding of the KKSN has helped Germany increase its clinical trial output.
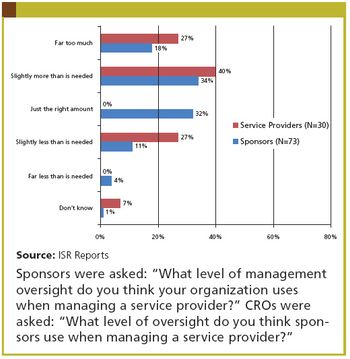
The perennial sponsor complaint: "Outsourcing costs too much." The perennial CRO response: "If you didn't assign so much internal resource to manage us, it would be cheaper."

Natural language processing could change the way we interpret documents and data.

The Middle East and North Africa region has seen a recent increase in clinical trials research.
Clinical trials may benefit from new "app" technology.