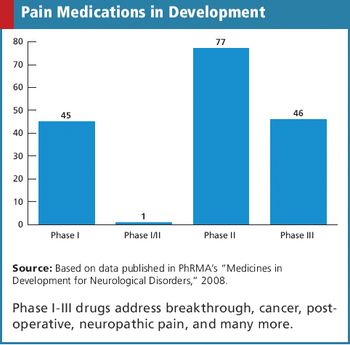
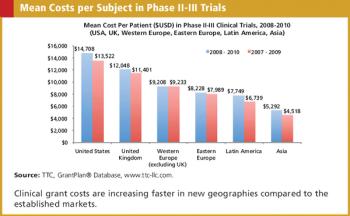
REGULATORY : Is It Safe to Outsource Safety? Waiving Inclusion/Exclusion Criteria CRO/SPONSOR : Metrics - The Pitfalls of Over Collection SUBJECT RECRUITMENT : Trial Results Provided to Volunteers in Pilot Program Also in this issue : New Reform Law Requires Fee Disclosure, GCP Standards in Global Trials, A Learning Healthcare System, The Physician Payments Sunshine Act