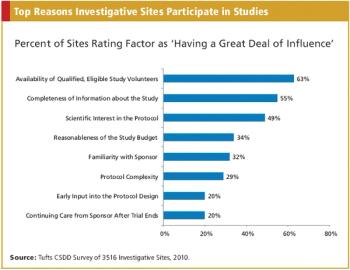
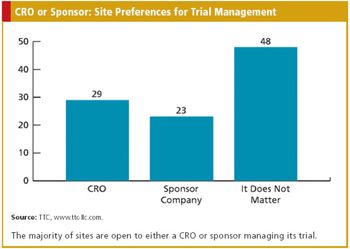
CRO/SPONSOR : The Nuances of Medical Device Trials A Triggered Approach to Site Monitoring INFORMATION TECHNOLOGY : Automating the Phase I Trial Also in this issue : FDA Addresses Concerns Over Foreign Studies, The Cost Factor in EU Drug Authorization, Baseball?s Ties to Comparative Effectiveness Research, Key Updates to the Form FDA 1572