
In this video interview, Rob Jones, product manager, TMF practice area, Pharmalex, shares his key takeaways from Cencora’s recent TMF Leadership Summit and touches on the importance of oversight when working with outside stakeholders.

In this video interview, Rob Jones, product manager, TMF practice area, Pharmalex, shares his key takeaways from Cencora’s recent TMF Leadership Summit and touches on the importance of oversight when working with outside stakeholders.

In this video interview, Rob Jones, product manager, TMF practice area, Pharmalex, discusses how risk-based approaches must be prioritized amidst the increased demand for quality and compliance.

Cobenfy (xanomeline and trospium chloride) as an adjunctive treatment to atypical antipsychotics demonstrated a 2.0-point reduction in PANSS total score in the ARISE study, failing to meet statistical significance.

In this video interview, Rob Jones, product manager, TMF practice area, Pharmalex, highlights recent ICH guidelines and how risk should be an area of focus as trial designs continue to get more complex.

In this video interview, Rob Jones, product manager, TMF practice area, Pharmalex, talks AI and how change is needed for the clinical trials industry to fully understand its potential with automation.

In this video interview, Rob Jones, product manager, TMF practice area, Pharmalex, discusses challenges the TMF space is currently facing and how it can utilize collaboration in making advancements.

The Phase II study, PROGRESS-AD, will evaluate the safety and efficacy of human monoclonal antibody AL101/GSK4527226 over 76 weeks.

In this video interview, Derek Ansel, vice president, therapeutic strategy lead, rare disease, Worldwide Clinical Trials, discusses how artificial intelligence can aid in bringing therapies to rare disease patients faster.

In addition to meeting the primary endpoint of superior A1C reduction, the once-daily oral pill reduced weight by an average of 16 lbs.

In this episode of the Applied Clinical Trials Podcast, Liz Beatty, co-founder and chief strategy officer, Inato, discusses a number of topics around site engagement including community-based sites, the role of technology in improving site/sponsor relationships, how increased operational costs are impacting the industry, and more.

In this video interview, Derek Ansel, vice president, therapeutic strategy lead, rare disease, Worldwide Clinical Trials, highlights the Rally for Rare event and how it succeeded in providing the community a space for collaboration and innovation.
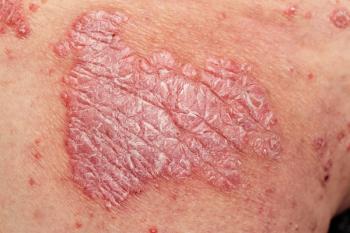
Results from a subgroup of the ICONIC-LEAD study show 75% of adolescents with plaque psoriasis achieved completely clear skin.

In this video interview, Derek Ansel, vice president, therapeutic strategy lead, rare disease, Worldwide Clinical Trials, talks collaboration and maximizing resources among the various stakeholders in rare disease research.

In this video interview, Derek Ansel, vice president, therapeutic strategy lead, rare disease, Worldwide Clinical Trials, discusses the most prominent challenges he is seeing in the space right now including competing research priorities and finding endpoints.

In this video interview, Thierry Escudier, portfolio lead, Pistoia Alliance, highlights the need for maintaining data privacy in a highly regulated clinical research industry.

In this video interview, Thierry Escudier, portfolio lead, Pistoia Alliance, discusses how an emphasis on patient centricity can encourage more sustainable trial designs.

In this video interview, Thierry Escudier, portfolio lead, Pistoia Alliance, talks the current landscape of sustainability in clinical trials and how stakeholders are beginning to pay more attention to carbon footprint amid increasing protocol complexity.

In this video interview, Thierry Escudier, portfolio lead, Pistoia Alliance, highlights where regulatory agencies currently stand on sustainability in clinical trials and how pharma companies are looking to reduce their carbon footprint.
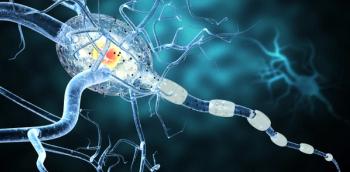
In the Phase III MUSETTE study, a higher dose of intravenous Ocrevus (ocrelizumab) did not provide additional benefit in slowing disability progression compared to the currently approved 600mg dose.

In this video interview, Thierry Escudier, portfolio lead, Pistoia Alliance, discusses the alliance’s latest step in evaluating the sustainability of digital versus traditional clinical trial approaches.

In this video interview, Craig Lipset, co-chair, Decentralized Trials & Research Alliance (DTRA), discusses numerous themes that clin ops can focus on to keep its trials running efficiently and effectively.

In this video interview, Craig Lipset, co-chair, Decentralized Trials & Research Alliance (DTRA), highlights how research communities have self-organized in light of recent changes.

The combination met its primary endpoint of percent weight loss following an 8-week treatment period.

In this video interview, Craig Lipset, co-chair, Decentralized Trials & Research Alliance (DTRA), discusses how companies in the clinical research industry have been responding to recent political headwinds.

Winrevair (sotatercept-csrk), compared to placebo, reduced the risk of all-cause death, lung transplantation, and hospitalization.

In this video interview, Craig Lipset, co-chair, Decentralized Trials & Research Alliance (DTRA), highlights how recent funding cuts are impacting critical research resources.

Subcutaneous administration of pembrolizumab with chemotherapy demonstrated a nearly 50% reduction in patient chair and treatment room time while maintaining efficacy and safety endpoints compared to intravenous Keytruda.

In this video interview, Craig Lipset, co-chair, Decentralized Trials & Research Alliance (DTRA), discusses how the current political climate is affecting diversity in clinical research and how diversity action plans may be impacted.
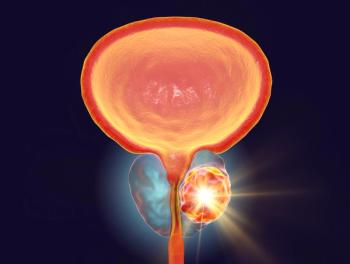
Based on additional results from the Phase III PSMAfore clinical trial, the therapy is now approved for use prior to chemotherapy in PSMA-positive metastatic castration-resistant prostate cancer.

In this video interview, Craig Lipset, co-chair, Decentralized Trials & Research Alliance (DTRA), shares his thoughts on the recent funding cuts made by the NIH and how they are impacting medical research.